

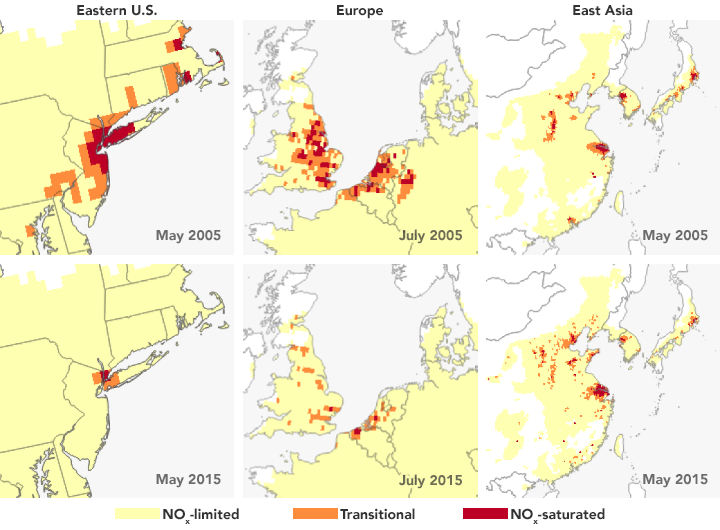
Ozone pollution near Earth’s surface is one of the main ingredients of summertime smog and a primary cause of poor air quality. Yet it is not directly measurable from space because of the abundance of ozone higher in the atmosphere, which obscures measurements of surface ozone. Now NASA-funded researchers have devised a way to use satellites to measure the precursor gases that contribute to ozone formation. By differentiating among three possible conditions that lead to ground-level ozone production, the observations may assist air quality managers in assessing the most effective approaches to emission reduction and air quality improvements.
At high altitude, ozone acts as Earth’s sunscreen from harmful ultraviolet radiation. At low altitudes, ozone is a health hazard contributing to respiratory problems like asthma and bronchitis. Near the ground, the gas is formed through complex chemical reactions initiated by sunlight and involving volatile organic compounds (VOCs) and nitrogen oxides (NOx). It turns out that formaldehyde (a VOC) and nitrogen dioxide (NO2), are measurable from space by the Dutch-Finnish Ozone Monitoring Instrument aboard NASA’s Aura satellite.
“We are using satellite data to analyze the chemistry of ozone from space,” said lead author Xiaomeng Jin of the Lamont-Doherty Earth Observatory. The research was published in the Journal of Geophysical Research: Atmospheres.
Through a combination of computer models and space-based observations, Jin and colleagues used the concentrations of the precursor molecules to infer whether ozone production at a given location increases more in the presence of NOx, VOCs, or a mix of the two. Their study regions focused on North America, Europe, and East Asia during the summer, when abundant sunlight triggers the highest rates of ozone formation. To understand their impact on ozone formation, the team investigated whether VOC or NOx was the ingredient that most limited ozone formation. If emissions of that molecule can be reduced, then ozone formation will be reduced—critical information for air quality managers.
“We are asking: ‘If I could reduce either VOCs or NOx, which one is going to get me the biggest bang for my buck in terms of the amount of ozone that we can prevent from being formed in the lower atmosphere?’” said co-author and atmospheric chemist Arlene Fiore of Lamont-Doherty, who is also a member of NASA’s Health and Air Quality Applied Sciences Team.
The researchers found that the urban regions that they studied (shown above) are more often VOC-limited or in a transitional state between VOC and NOx-limited. Looking at 12 years of Aura measurements, they also found that circumstances can change. For instance, New York City’s ozone production in the summer of 2005 was limited by VOCs; by 2015, it had transitioned to a NOx-limited system thanks to pollution controls put into place at regional and national levels. This transition means future NOx reductions will likely further decrease ozone production, Jin said.
Volatile organic compounds occur naturally; they are most often emitted by trees in the form of formaldehyde. They can also arise from paint fumes, cleaning products, and pesticides, and they are a by-product of burning fossil fuels in factories and automobiles. Nitrogen oxides are a byproduct of burning fossil fuels and are abundant in cities, where they are emitted by power plants, factories, and cars. Because VOCs have a large natural source (trees) in the eastern United States, for example, emission reduction plans have focused on NOx, which is overwhelmingly produced by human activities and therefore more controllable.
NASA Earth Observatory image by Joshua Stevens, using data from Jin, Xiaomeng, et al. (2017). Story by Ellen Gray, NASA’s Earth Science News Team, with Mike Carlowicz, Earth Observatory.